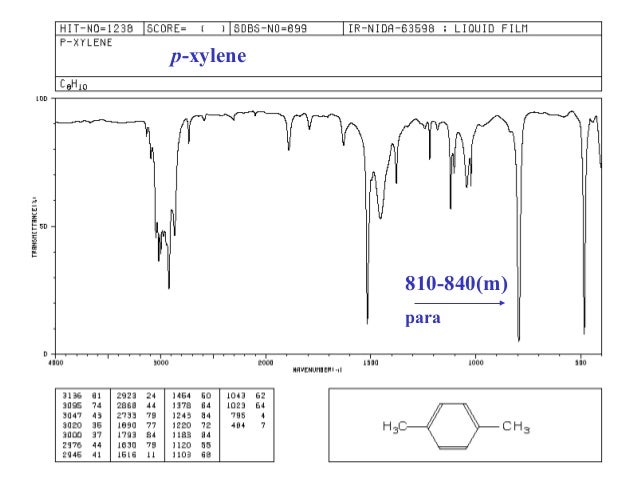
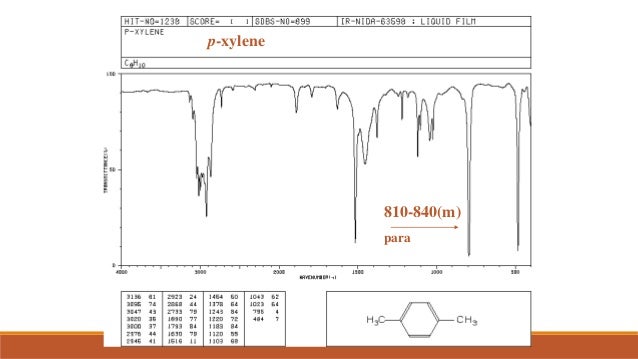
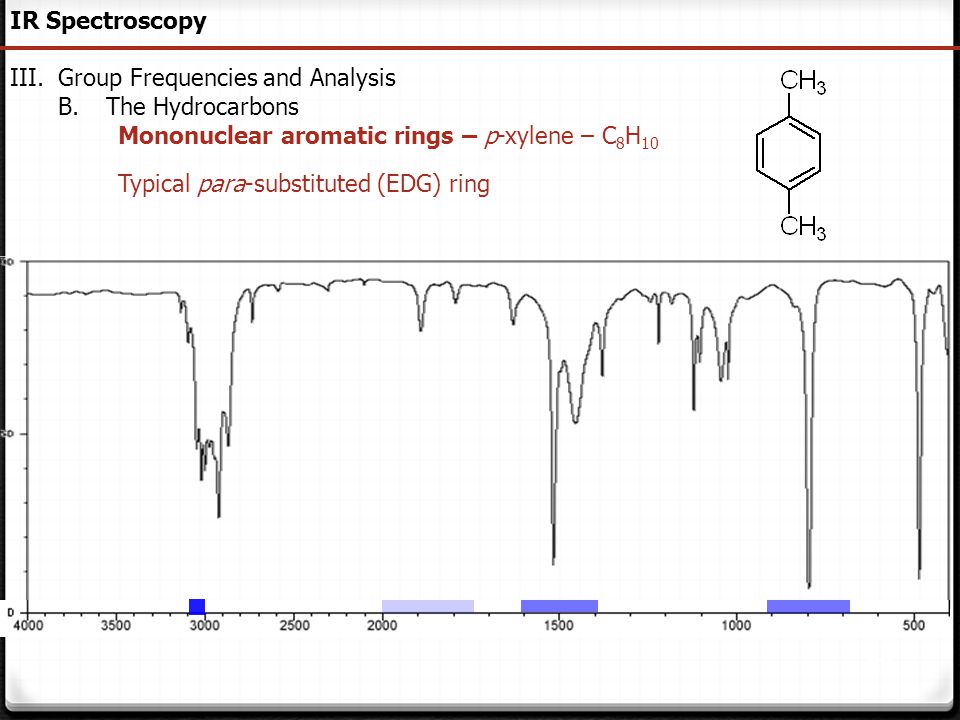
P-xylene ir spectrum labeled 472439-P-xylene ir spectrum labeled
DIGITIZED BY NIST FROM HARD COPY (FROM TWO SEGMENTS);Li et al, 17;Go To Top, Infrared Spectrum, References Data from NIST Standard Reference Database 69 NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment

Organic Spectroscopy International Ir Spectra Examples
P-xylene ir spectrum labeled
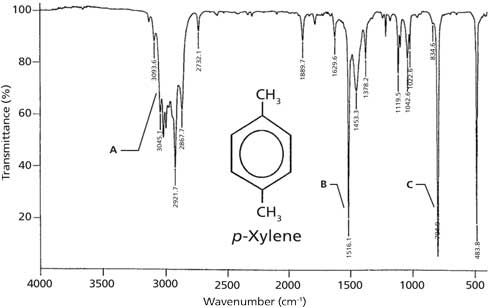
P-xylene ir spectrum labeled-View the Full Spectrum for FREE!PXylene labeled to colaborate with bond length table Table 1 Bond lengths of pXylene Basis Type Bond Length Measurments (pm) Bonds (pm) 621G This IR spectrum 2 was used to determine what vibrational configurations occurred most often These configurations are listed and presented below


4
Ma et al, 18), while the peaks at m/z 95 and 77 under highNO conditions (Fig 2, Fig 3) are tentatively assigned to protonated phenol and its dehydrated formThe full spectrum can only be viewed using a FREE accountDIGITIZED BY NIST FROM HARD COPY (FROM TWO SEGMENTS);
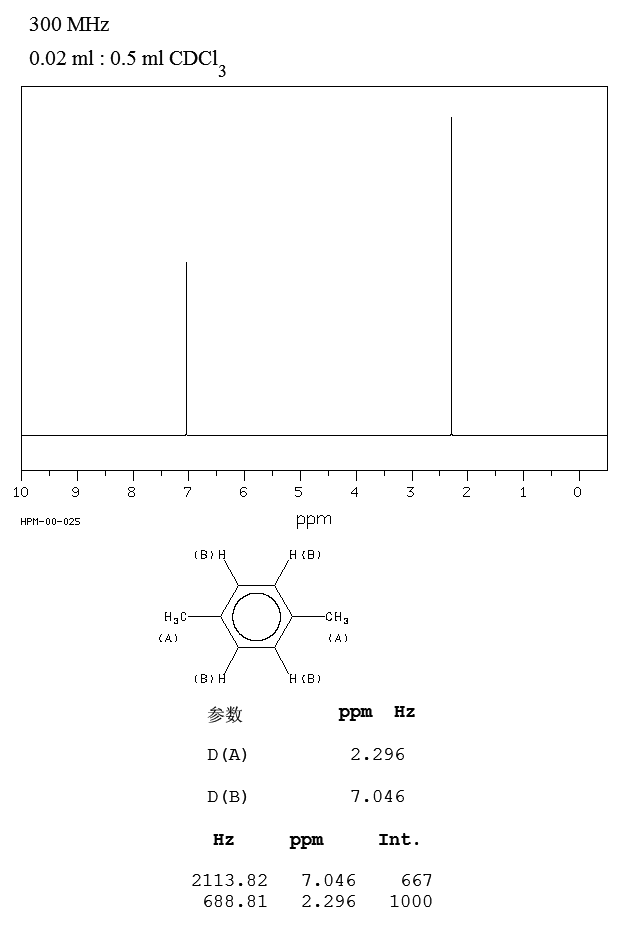
Humans exposed to 46 or 92 ppm of o, m, pxylene or a mixture (111) of the three for 8 hr absorbed approx 64% of the inhaled xylene No difference in the absorption rate was reported due to level of exposure, length of exposure, or the type and/or mixture of the xylene isomersThe 1 H NMR spectrum of 1,4dimethylbenzene (pxylene), shown in Figure below, is a simple example that we can use to learn how to interpret chemical shifts First, note that there is a signal at δ 0View the Full Spectrum for FREE!
PXylene reference substance for gas chromatography Synonym 1,4Dimethylbenzene, pXylene CAS Number Linear Formula C 6 H 41,4(CH 3) 2 Molecular Weight MDL number MFCD EC Index NumberThe peak at m/z 121 observed in the spectrum of pxylene SOA (Figs 2A–1 and B1) is tentatively assigned to protonated 4methylbenzaldehyde (Bloss et al, 05;10) Comment on the appearance of both the interferogram and the spectra in both cases Salt plates, liquid cell If actual liquid cell is not available, sandwich 2 salt plates together a Collect a spectrum of neat oxylene, mxylene and pxylene (remember, IR is very


P Xylene



Media Portfolio
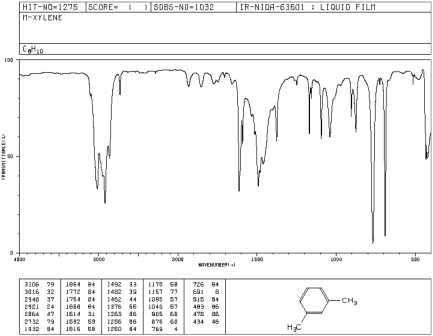
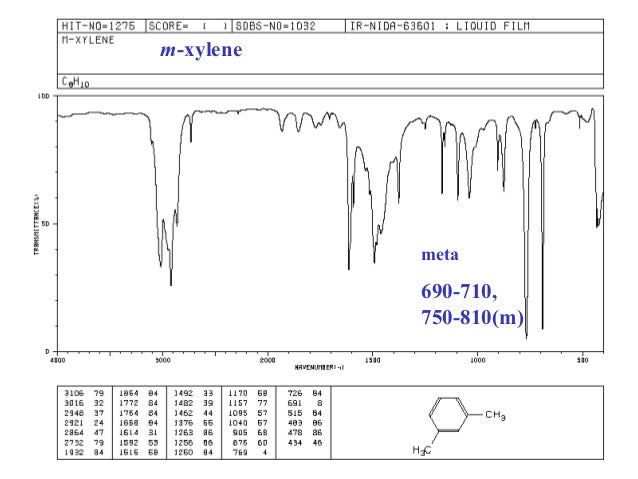
Commercial or mixed xylene usually contains about 4065% mxylene and up to % each of o and pxylene and ethylbenzene (1) Mixed xylenes are colorless liquids that are practically insoluble in water and have a sweet odor (1) The odor threshold for mxylene is 11 ppm (4)An infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation isThe oop bends are sometimes useful in distinguishing substitution pattrens around a benzene ring Using the spectra of o, m, and pxylene, formulate some guidelines about what the oop bends look like when substituents are one, two or three carbons away on a benzene ring Figure IR18 IR spectrum of mxylene
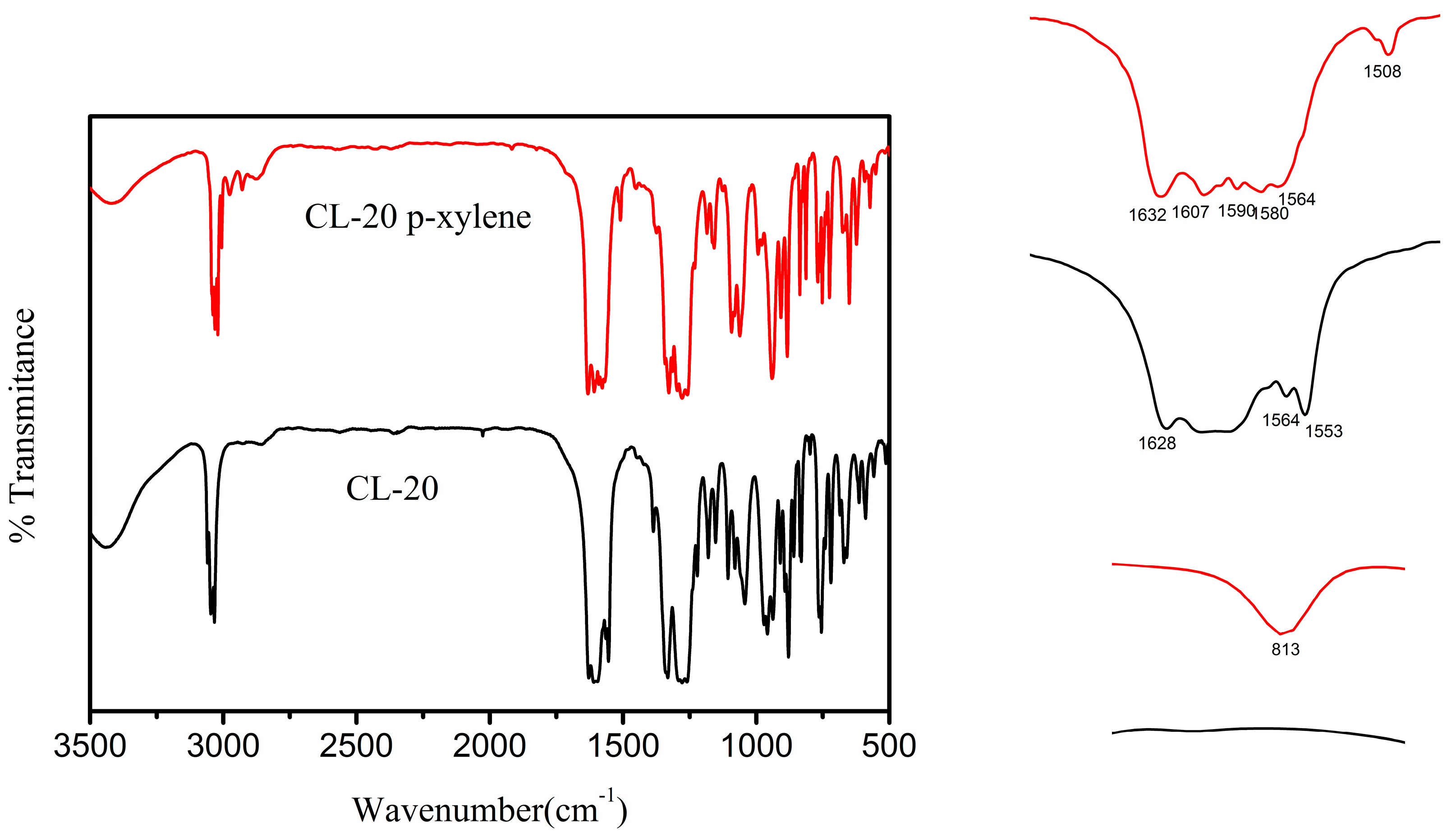


Molecules Free Full Text The Crystal Structure And Morphology Of 2 4 6 8 10 12 Hexanitro 2 4 6 8 10 12 Hexaazaisowurtzitane Cl P Xylene Solvate A Joint Experimental And Simulation Study Html


Http Www Ifsc Usp Br Lavfis2 Bancoapostilasimagens Apluminescencia Infrared spectroscop1 Pdf
Table 1 Principal IR Absorptions for Certain Functional Groups Functional Group Names & Example compounds Absorption Ranges(cm1) Look for a single absorption in these regions, unless stated otherwise Type of Vibration causing IR absorption Esters C=O Stretch H C O O CH 3 Methyl Formate () (CO Stretch) Ethers O DiethylThe full spectrum can only be viewed using a FREE accountExpert Answer 100% (1 rating) Previous question Next question



3 2 Ir Spectroscopy Chemistry Libretexts



Spectroscopy And Structure Mcgraw Hill Education Access Engineering
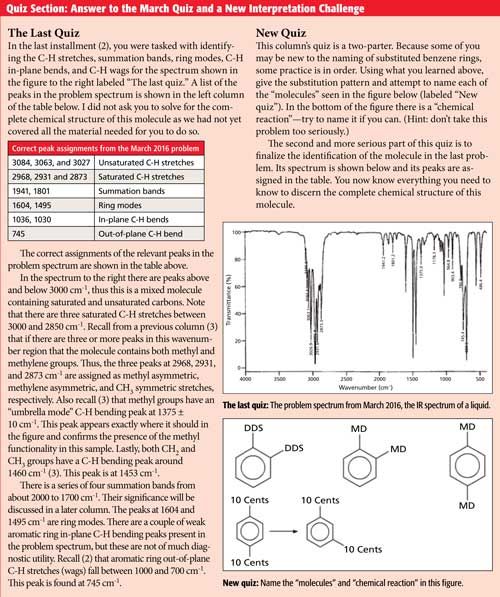
Go To Top, Infrared Spectrum, References Data from NIST Standard Reference Database 69 NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgmentWhen analyzing an IR spectrum, it is helpful to overlay the diagram below onto the spectrum with our mind to help recognize functional groups Figure 1 Group frequency and fingerprint regions of the midinfrared spectrum The region of the infrared spectrum from 10 to 700 cm1 is called the fingerprint region This region is notable for theM, o, and pXylene are the three isomers of xylene;


Formation Kinetics And Photoelectrochemical Properties Of Crystalline C70 One Dimensional Microstructures Rsc Advances Rsc Publishing


Micro Transflection On A Metallic Stick An Innovative Approach Of Reflection Infrared Spectroscopy For Minimally Invasive Investigation Of Painting Varnishes Springerlink
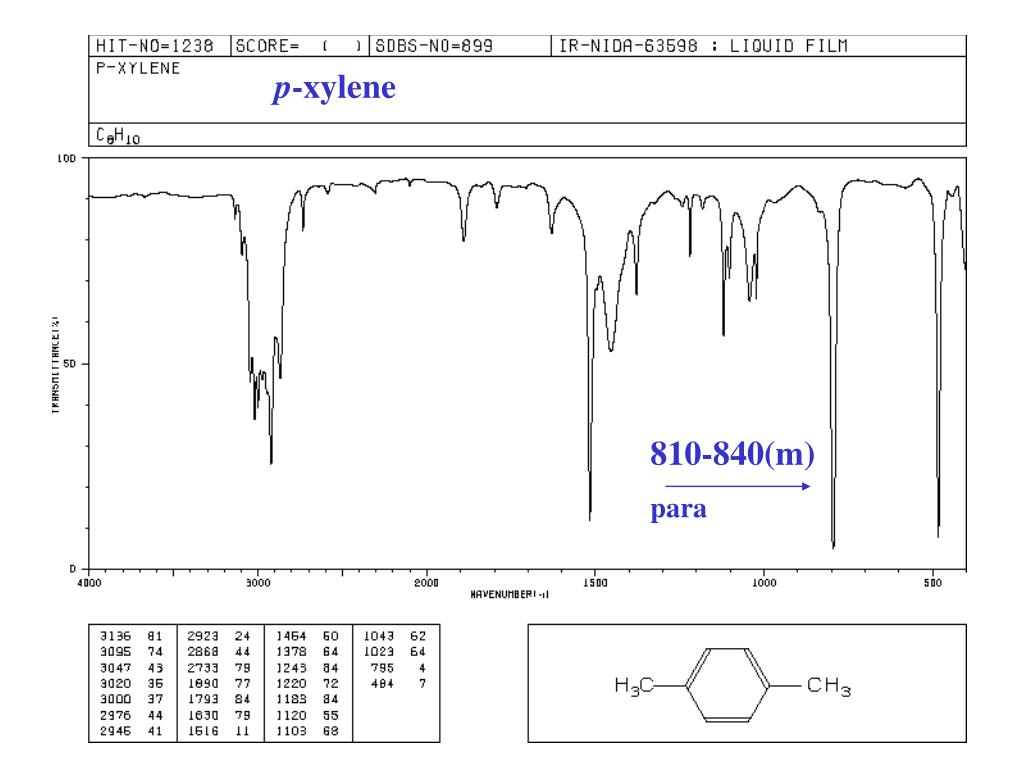
Structure and properties Index of refraction, n D at °C Dielectric constant, ε r 22 ε 0 at °C Surface tension 2992 dyn/cm at 5 °C 27 dyn/cm at °C1 Consider the three IR spectra below for three xylene isomers (o, m or pxylene) Label each IR spectrum with the structure of the xylene isomer that it corresponds to 100 80 60 40 Wavenumber (cm) 100 80 8 60 40 Wavenumber (cm) 100 80 60 40 0 Wavenumber (cm)Alkyl halides are compounds that have a C–X bond, where X is a halogen bromine, chlorine, fluorene, or iodine (usually Br or Cl in the organic chemistry teaching labs) In general, C–X vibration frequencies appear in the region cm1, sometimes out of the range of typical IR instrumentation


Infrared Spectroscopy



Isotope Effects In Liquid Water By Infrared Spectroscopy V A Sea Of Oh4 Of C2v Symmetry The Journal Of Chemical Physics Vol 134 No 16
Go To Top, Mass spectrum (electron ionization), References Data from NIST Standard Reference Database 69 NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound13CNMR and IR spectra appear on pages 8 and 9 (B) Label the appropriate protons on your deduced structure to correspond to each set of signals in the 1HNMR spectrum (C) Label proper groups of the structure that are responsible for the circled peaks in the IR spectrum 2Precautionary Statement Codes P210, P233, P240, P241, P242, P243, P280, P303P361P353, P370P378, P403P235, and P501 (The corresponding statement to each Pcode can be found at the GHS Classification page) ECHA C&L Notifications Summary
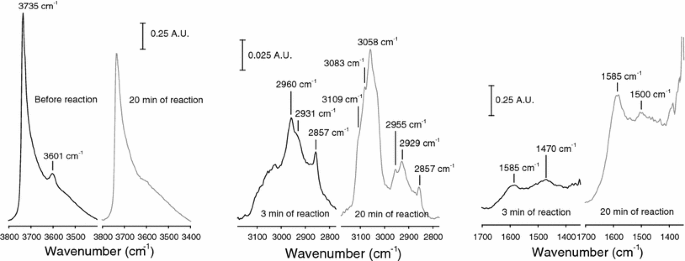


In Situ Ft Ir Mechanistic Investigations Of The Zeolite Catalyzed Methylation Of Benzene With Methanol H Zsm 5 Versus H Beta Springerlink


Www Studocu Com En Us Document University Of Alabama At Birmingham Organic Chemistry Ii Lab Essays Diels Alder Reaction Of Anthracene And Maleic Anhydride View
This spectrum contains signals from all there xylene isomers and the ethylbenzene impurity Each isomer of xylene produces a slightly different 1H NMR spectrum The highly symmetric pxylene produces two signals, one aliphaticSearch results for pXylol at SigmaAldrich Compare Products Select up to 4 products *Please select more than one item to comparePXylene, Reagent is a highly flammable aromatic hydrocarbon that is used mainly as a solvent The Reagent grade denotes that this chemical is the highest quality commercially available and that the American Chemical Society has not offi



Pyridinium Protic Ionic Liquids Effective Solvents For Delignification Of Wheat Straw Sciencedirect


Http Www1 Udel Edu Chem Fox Ir Lecturenotes Pdf
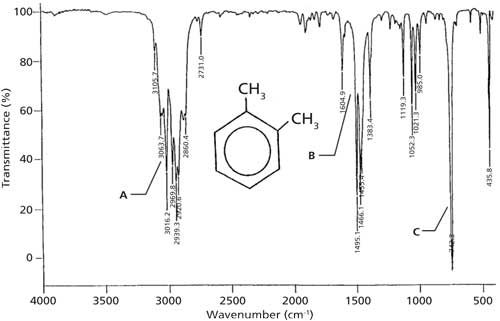
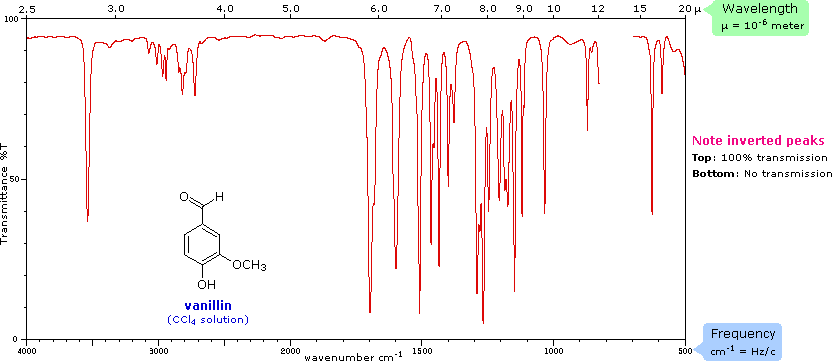
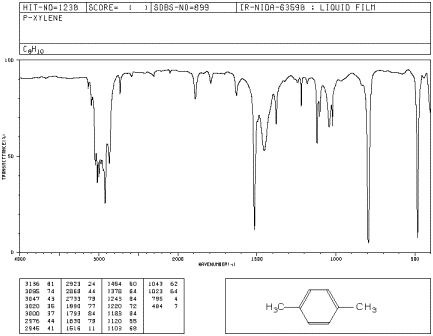
PXYLENE State SOLUTION (10% IN CCl4 FOR , 10% IN CS2 FOR , AND 10% CCl4 FOR CM1) VERSUS SOLVENT Instrument This IR spectrum is from the Coblentz Society's evaluated infrared reference spectra collection References Go To Top, Infrared Spectrum, NotesCompound pXylenewith free spectra 60 NMR, 19 FTIR, 2 Raman, 2 Near IR, and 23 MSQuantitative Investigations of Biodiesel Fuel Using Infrared Spectroscopy An Instrumental Analysis Experiment for Undergraduate Chemistry Students Journal of Chemical Education 12 , (2) ,


Distinguishing Structural Isomers Mono And Disubstituted Benzene Rings



Media Portfolio
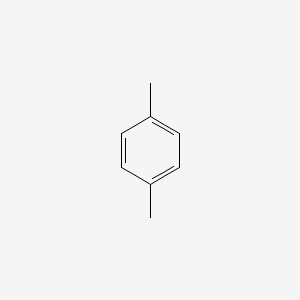
2,5Dichloropxylene C8H8Cl2 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activitiesPXylene (paraxylene) is an aromatic hydrocarbonIt is one of the three isomers of dimethylbenzene known collectively as xylenesThe pstands for para, indicating that the two methyl groups in pxylene occupy the diametrically opposite substituent positions 1 and 4It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, oIts IR spectrum also showed a strong absorption at 1667 cm1 indicating the presence of a carbonyl group The melting point of the recovered fraction was °C (matches the documented value of 81 – °C), confirming that the second fraction was monoacetylated ferrocene
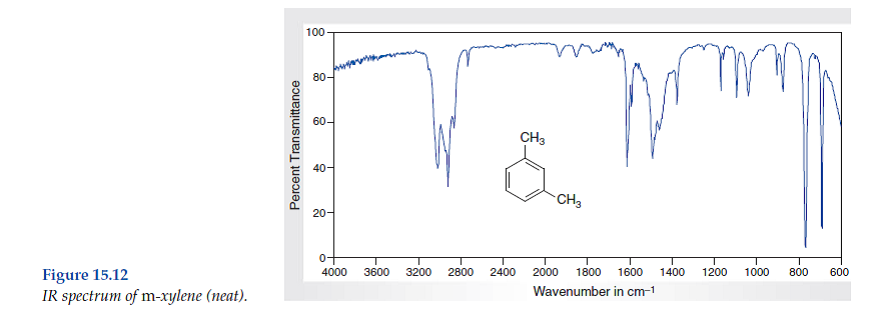


Solved Consider The Spectral Data For M Xylene Figs 15 12 An Chegg Com


4
Your source of analytical, research, development, and productionquantity fine chemicals for nearly 40 yearsThe full spectrum can only be viewed using a FREE accountGeneral description pXylened 10 is a deuterated derivative of pxyleneIt has an isotopic purity of 98atom%D A series of solutions of pxylene dissolved in the first 12 members of the 4nalkyloxy4′cyanobipheny ls (NOCB) were prepared Principal components S zz and S xx S yy of the second rank orientational ordering matrix of these solutions were determined by deuterium NMR analysis
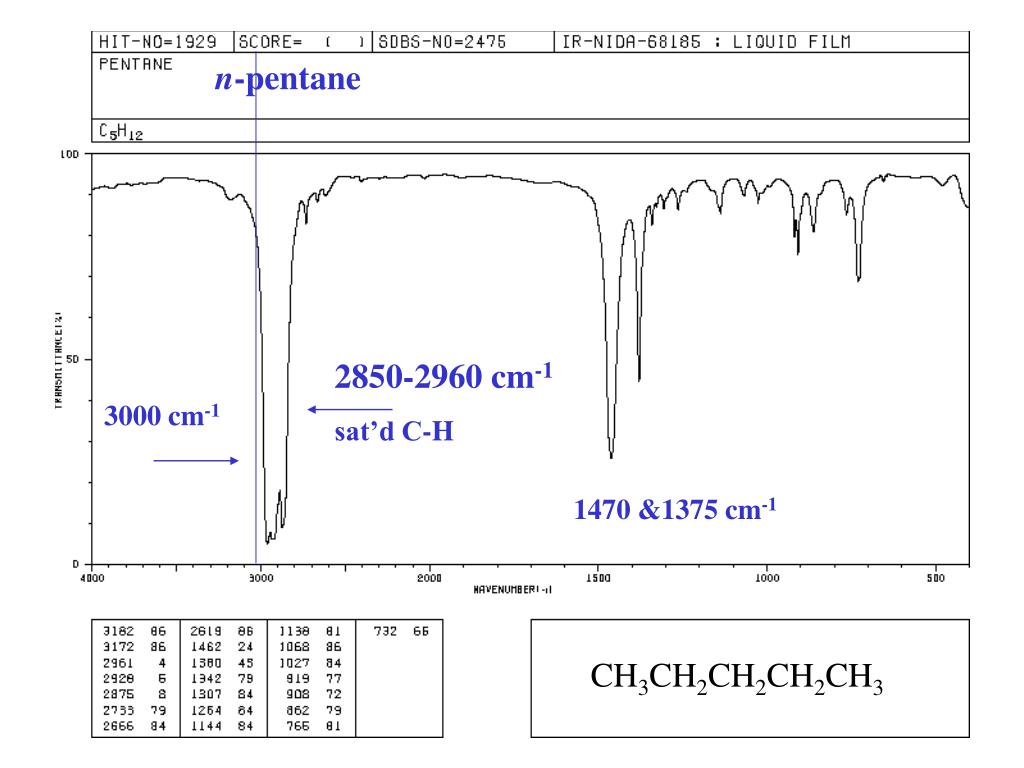


Ppt Spectroscopy Infrared Spectra Powerpoint Presentation Free Download Id


Www Jstor Org Stable
Commercial or mixed xylene usually contains about 4065% mxylene and up to % each of oxylene and pxylene and ethylbenzeneXylenes are released into the atmosphere as fugitive emissions from industrial sources, from auto exhaust, and through volatilization from their use as solventsMaterial Safety Data Sheet The handling of this chemical may incur notable safety precautions It is highly recommend that you seek the Material Safety Datasheet for this chemical from a reliable source and follow its directionsMATHESON TRIGAS, INCFrom the IR spectrum organic chemistry lab How can mxylene be distinguished from pxylene using IR spectroscopy?


Infrared Spectroscopy



Download List Of Spectra Ftir Spectra Infrared Spectra Library
In rats and mice, m and pxylene are distributed primarily to lipidrich tissues, such as fat, blood, and brain and also in organs highly perfused with blood such as kidney and liver Small amounts of pxylene and oxylene cross the placenta and distribute to amnionic fluid and fetal tissue Oral administration of mxylene to rats led toThe oop bends are sometimes useful in distinguishing substitution pattrens around a benzene ring Using the spectra of o, m, and pxylene, formulate some guidelines about what the oop bends look like when substituents are one, two or three carbons away on a benzene ring Figure IR18 IR spectrum of mxyleneThe small mass peak at 107 amu corresponds to 13 Clabeled pxylene, where one 13 C atom replaces a carbon of pxyleneThe natural abundance of 13 C is 1108% In pxylene, however, the natural abundance of 13 Clabeled molecules amounts to more than 8% due the presence of eight carbon atoms in molecule The observed ratio of intensities of two peaks in Fig 1 is in agreement with the natural



An Overview On Recent Advances In The Synthesis Of Sulfonated Organic Materials Sulfonated Silica Materials And Sulfonated Carbon Materials And Their Catalytic Applications In Chemical Processes Abstract Europe Pmc


Q Tbn And9gcs2kgpc0z0h01gzmkeg Fbvoz05czgru Fez2o2tqeqx9t7wq9a Usqp Cau
SOLUTION (10% IN CCl4 FOR , 10% IN CS2 FOR , AND 10% CCl4 FOR CM1) VERSUS SOLVENT;An infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation isPURE CULTURE A Pseudomonas strain, isolated from soil, was able to utilize 4methylbenzaldehyde as the sole carbon source, giving 018, 027, and 043 um O2 consumed/min/mg dry weight cells, for cells previously grown on mxylene, pxylene, and pmethylbenzyl alcohol, respectively(1)


P Xylene


Ir Problems
Spectrum Chemical Quality and delivery you can count on every time!View the Full Spectrum for FREE!View Expt2_IR_v13_Shortenedpdf from CHEM 2350 at The Hong Kong University of Science and Technology CHEM2350 Analytical Chemistry Lab Quantitative Analysis of Xylene Isomers (mXylene and pXylene)


P Xylene 106 42 3 1h Nmr


Distinguishing Structural Isomers Mono And Disubstituted Benzene Rings
IR Spectrum Go To Top, References, Notes Data compiled by Coblentz Society, Inc LIQUID;The small mass peak at 107 amu corresponds to 13 Clabeled pxylene, where one 13 C atom replaces a carbon of pxyleneThe natural abundance of 13 C is 1108% In pxylene, however, the natural abundance of 13 Clabeled molecules amounts to more than 8% due the presence of eight carbon atoms in molecule The observed ratio of intensities of two peaks in Fig 1 is in agreement with the naturalThe oop bends are sometimes useful in distinguishing substitution pattrens around a benzene ring Using the spectra of o, m, and pxylene, formulate some guidelines about what the oop bends look like when substituents are one, two or three carbons away on a benzene ring Figure IR18 IR spectrum of mxylene


How Could Ir Spectroscopy Be Used To Distinguish Between Benzene And Cyclohexene Socratic
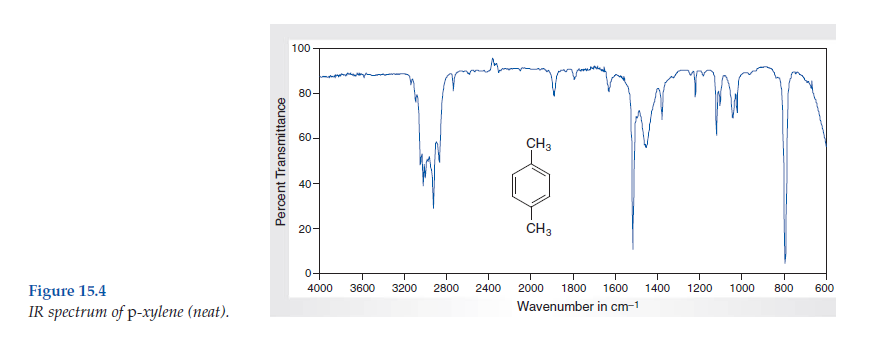


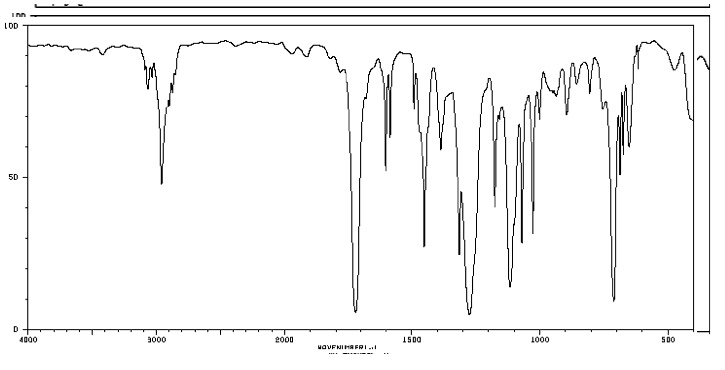
Solved Consider The Spectral Data For P Xylene Figs 15 4 And Chegg Com
PXylene View entire compound with free spectra 60 NMR, 19 FTIR, 2 Raman, 2 Near IR, and 23 MS SpectraBase Compound ID Molecular Formula C8H10 Exact Mass g/mol Transmission Infrared (IR) Spectrum View the Full Spectrum for FREE!When done with the experiment, empty your 25 mL volumetric flasks into the bottle in the hood labeled WASTE CYCLOHEXANE AND XYLENES V IR spectra modeling using HyperChem 51 Using SGI 3 computer, open HyperChem 51 Load appropriate file ("oxylene", "pxylene", etc) Model the IR spectra and compare with the experimental data1,4Dibromo2,5bis(bromomethyl)benzene, among Spectrum Chemical's wide selection of high quality research chemicals, is also known as ,,2,5Tetrabromopxylene or 1,2,4,5tetrabormo3,6dimethylbenzen


Http Etemkose Cbu Edu Tr Wp Content Uploads 16 02 Ft Ir And Ft Raman Nmr And Uv Spectroscopic Investigation And Hybrid Computational Hf And Dft Analysis On The Molecular Structure Of Mesitylene 13 Pdf


Canvas Wisc Edu Courses Files Download Wrap 1
The 1 H NMR spectrum of 1,4dimethylbenzene (pxylene), shown in Figure below, is a simple example that we can use to learn how to interpret chemical shifts First, note that there is a signal at δ 0PXylene labeled to colaborate with bond length table Table 1 Bond lengths of pXylene Basis Type Bond Length Measurments (pm) Bonds (pm) 621G This IR spectrum 2 was used to determine what vibrational configurations occurred most often These configurations are listed and presented belowPXylene labeled to colaborate with bond length table Table 1 Bond lengths of pXylene Basis Type Bond Length Measurments (pm) Bonds (pm) 621G This IR spectrum 2 was used to determine what vibrational configurations occurred most often These configurations are listed and presented below


Http Www Ifsc Usp Br Lavfis2 Bancoapostilasimagens Apluminescencia Infrared spectroscop1 Pdf



Evolution Of The Ir Spectra Of P Xylene Measured Under High Pressure Download Scientific Diagram
Consider the spectral data for pxylene (Figs 154 and 155) a In the functional group region of the IR spectrum, specify the absorption associated with the πbonds of the aromatic ring Indicate with what structural feature the strong band at about 800 cm1 is associated b


Ppt Spectroscopy Infrared Spectra Powerpoint Presentation Free Download Id


A Web Page Containing Jmol Applets


How To Read Ir Spectroscopy Organic Chemistry Tutorials Youtube



Evolution Of The Ir Spectra Of P Xylene Measured Under High Pressure Download Scientific Diagram


Ir Spectra For Hydrocarbons Video Khan Academy


Www Osti Gov Servlets Purl


Computer Aided Pattern Recognition Of Organic Infrared Spectra Infrared And Raman Discussion Group Irdg
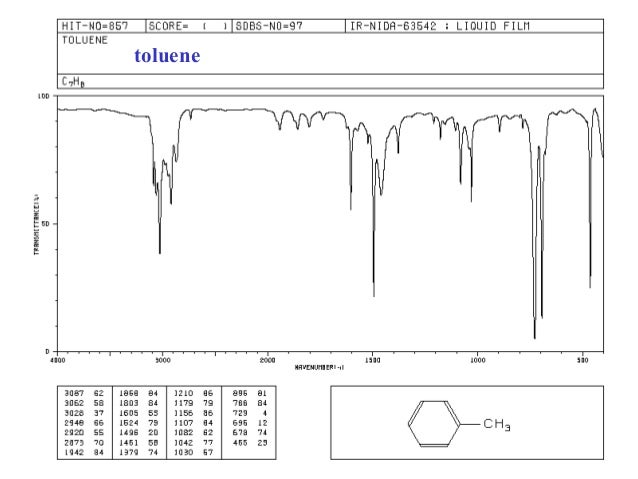


Infrared Spectroscopy



Separation And Isomerization Of Xylenes Using Zeolite Membranes A Short Overview Daramola 10 Asia Pacific Journal Of Chemical Engineering Wiley Online Library


P Xylene


How Would You Distinguish Between Ortho And Para Nitrophenol Using Infrared Spectroscopy Socratic


Canvas Wisc Edu Courses Files Download Wrap 1
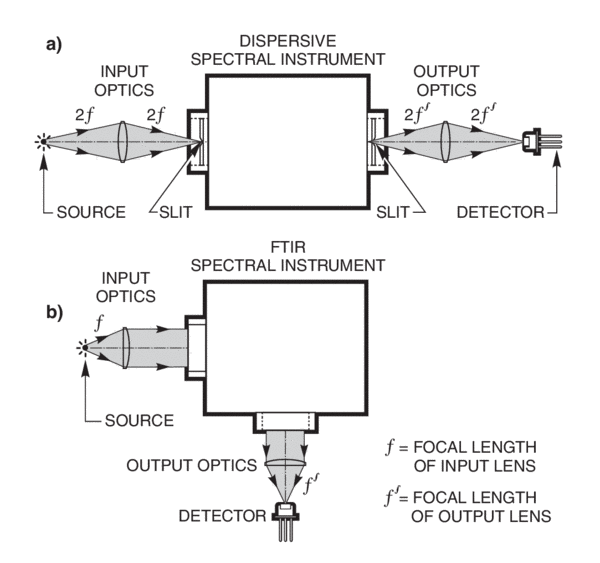


Ft Ir Spectroscopy


Www Perkinelmer Com Lab Solutions Resources Docs App Polymeridentificationmidinfaredspectroscopy Pdf



P Xylene Ftir Spectrum Spectrabase


Nanopdf Com Download Analyzing Structure Functions Infrared Spectra Pdf


Infrared Spectroscopy
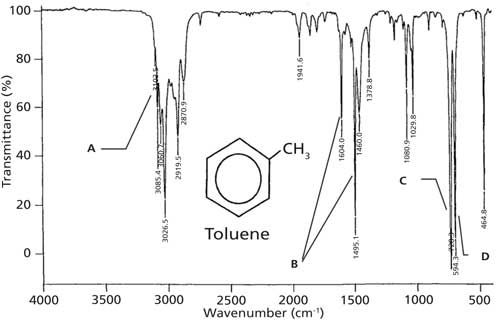


Distinguishing Structural Isomers Mono And Disubstituted Benzene Rings


How Would You Distinguish Between Ortho And Para Nitrophenol Using Infrared Spectroscopy Socratic



Organic Spectroscopy International Nuclear Magnetic Resonance Spectroscopy Nmr Spectroscopy



Ir Aromatics



Organic Spectroscopy International Ir Spectra Examples



Spectroscopy And Structure Mcgraw Hill Education Access Engineering


Infrared Spectroscopy


P Xylene Ftir Spectrum Spectrabase



Infrared Spectroscopy Electromagnetic Spectrum Ppt Download
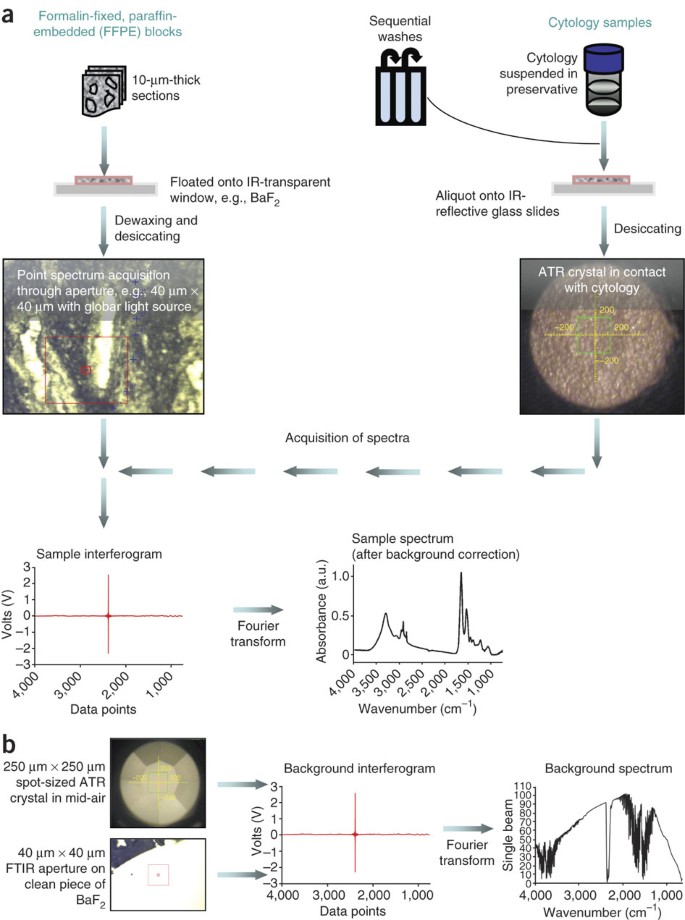


Distinguishing Cell Types Or Populations Based On The Computational Analysis Of Their Infrared Spectra Nature Protocols


Infrared Spectroscopy


Dspace Mit Edu Bitstream Handle 1721 1 1241 Doi 10 1142 s Pdf Sequence 2 Isallowed Y
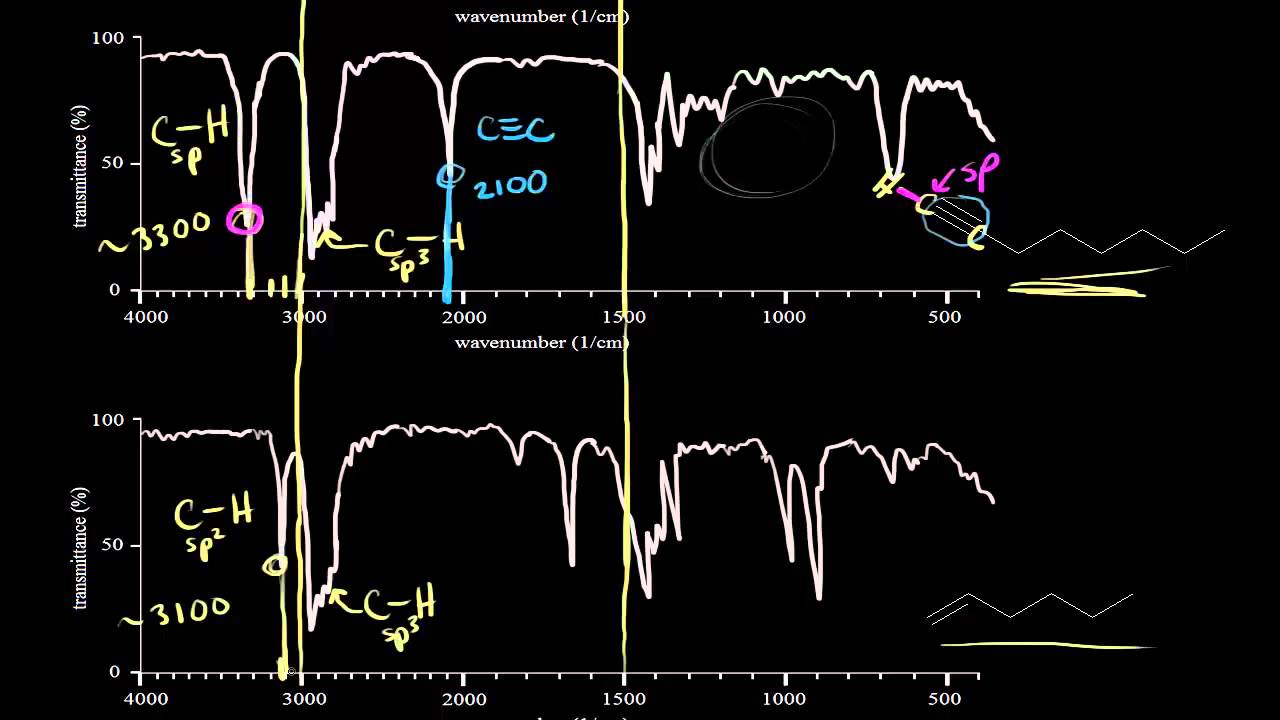


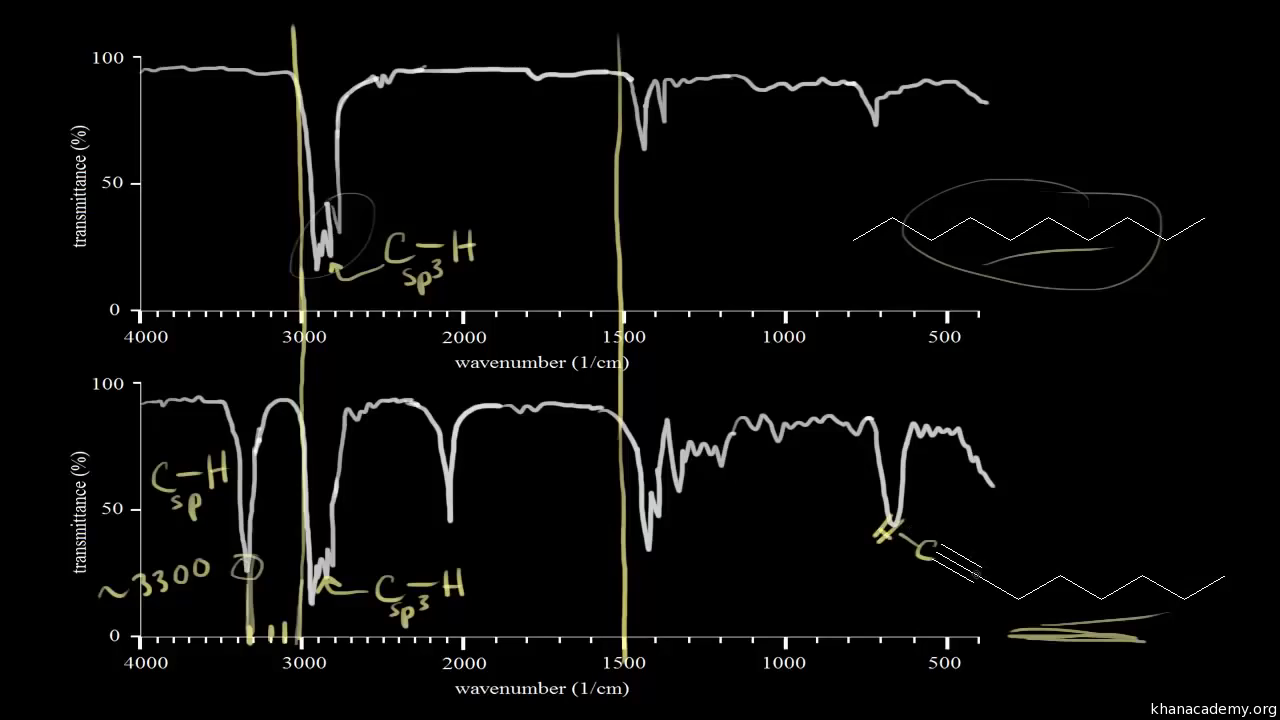
Ir Spectra For Hydrocarbons Video Khan Academy


Chem 430 Ir Spectroscopy Fall 11 Long Lecture Play Dr Justik Ppt Download


2 Bromo P Xylene C8h9br Pubchem
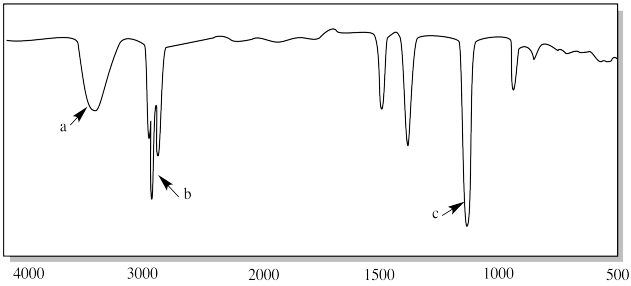


Ir Problems


Ir Spectra Of Selected Compounds Chemistry Libretexts



Selective Methylation Of Toluene Using Co2 And H2 To Para Xylene Science Advances


Dspace Mit Edu Bitstream Handle 1721 1 1241 Doi 10 1142 s Pdf Sequence 2 Isallowed Y
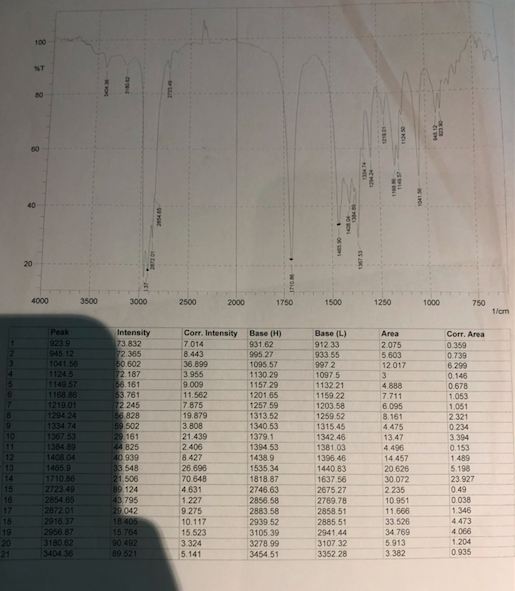


Solved Given The Ir Spectra Determine The Functional Gro Chegg Com


Osa Quantitative Analysis Of Essential Oils Of Thymus Daenensis Using Laser Induced Fluorescence And Raman Spectroscopy
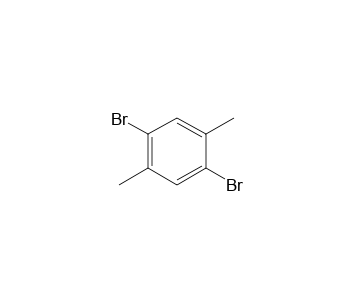


2 5 Dibromo P Xylene Vapor Phase Ir Spectrum Spectrabase


Infrared Spectroscopy


Idea Library Drexel Edu Islandora Object Idea 3a3192 Datastream Obj Download Preignition And Autoignition Behavior Of The Xylene Isomers Pdf



Improved Assignments Of The Vibrational Fundamental Modes Of Ortho Meta And Para Xylene Using Gas And Liquid Phase Infrared And Raman Spectra Combined With Ab Initio Calculations Quantitative Gas Phase Infrared Spectra For Detection



Selective Methylation Of Toluene Using Co2 And H2 To Para Xylene Science Advances


Chemengineering Free Full Text Synthesis Of Uniform Mesoporous Zeolite Zsm 5 Catalyst For Friedel Crafts Acylation Html


Http Journals Sagepub Com Doi Pdf 10 1366


Www Stolaf Edu People Hansonr Chem248 Reusch Organic problems Pdf



Improved Assignments Of The Vibrational Fundamental Modes Of Ortho Meta And Para Xylene Using Gas And Liquid Phase Infrared And Raman Spectra Combined With Ab Initio Calculations Quantitative Gas Phase Infrared Spectra For Detection


Q Tbn And9gcsc Waxi2fkicwyb2ewee2bh1sgy19al0qhv5xx2ox13nic N2a Usqp Cau


Ppt Spectroscopy Infrared Spectra Powerpoint Presentation Free Download Id



Functionalization Of Poly Para Xylylene S Opportunities And Challenges As Coating Material Moss Advanced Materials Interfaces Wiley Online Library
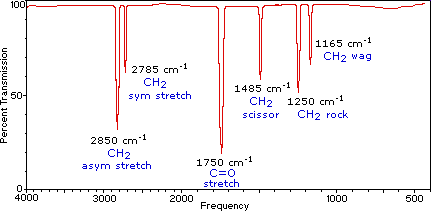


Infrared Spectroscopy


P Xylene C8h10 Chemspider


Http Www1 Udel Edu Chem Fox Ir Lecturenotes Pdf


Http Www Ifsc Usp Br Lavfis2 Bancoapostilasimagens Apluminescencia Infrared spectroscop1 Pdf



Organic Chemistry 332 Sapling Learning Ch 14 Flashcards Quizlet


Http Www Chemistry Uoc Gr Lapkin Infrared And Raman Spectroscopy Principles And Spectral Interpretation Pdf



Ir Of Synthesized Acetaminophen Use The Ir Spectrum Of Your Synthesized Acetaminophen To Answer The Homeworklib
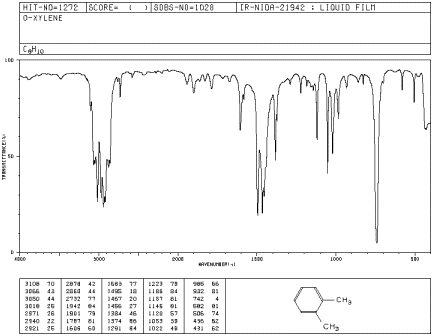


Ir Problems



Improved Assignments Of The Vibrational Fundamental Modes Of Ortho Meta And Para Xylene Using Gas And Liquid Phase Infrared And Raman Spectra Combined With Ab Initio Calculations Quantitative Gas Phase Infrared Spectra For Detection


Standard And Rapid Scan Infrared Spectroscopic Studies Of O Xylene Transformations In Terms Of Pore Arrangement Of 10 Ring Zeolites 2d Cos Analysis Dalton Transactions Rsc Publishing


M Xylene C6h4 Ch3 2 Pubchem


Q Tbn And9gcrpaaqeevd2m Qyvntd1ogzz7momhyzs1vohol4eadwvh8xaxj4 Usqp Cau


Q Tbn And9gcrlug Xgxgmotzt9ymoy 8rypk Z U4lkbnkvqky4jgu0bxdl8u Usqp Cau


Distinguishing Structural Isomers Mono And Disubstituted Benzene Rings


4


Ir Problems



Ftir Spectrum Of Urea Adducts With M Nitrobenzoic Acid M Nitroaniline Download Scientific Diagram


P Xylene C6h4 Ch3 2 Pubchem


Ir Problems
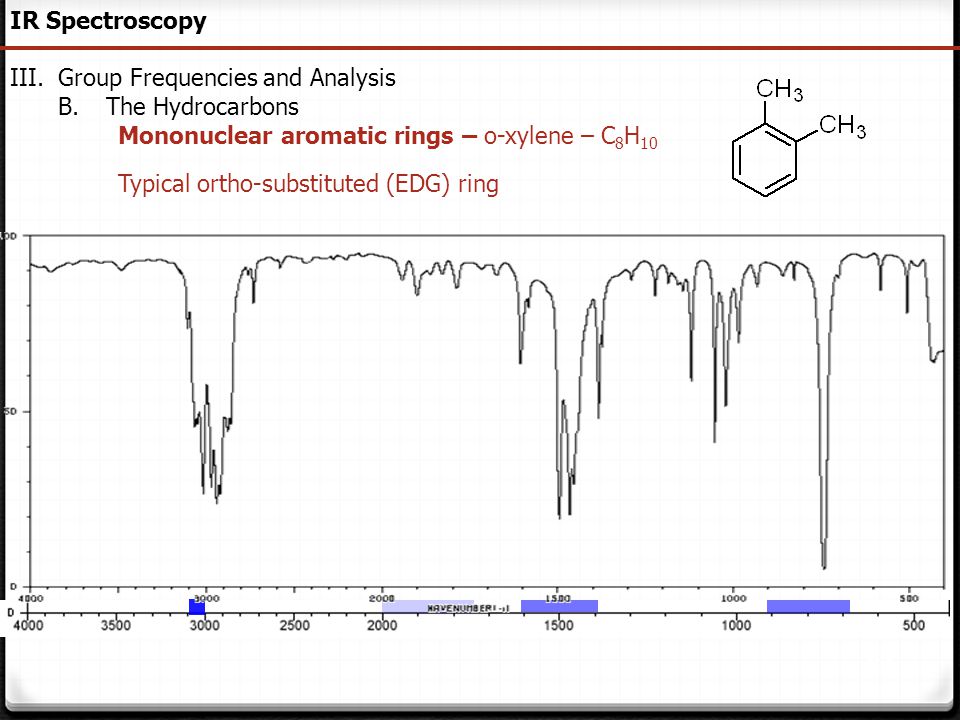


Chem 430 Ir Spectroscopy Fall 11 Long Lecture Play Dr Justik Ppt Download


コメント
コメントを投稿